Source: WHO Global Surveillance and Monitoring System for substandard and falsified medical products, Geneva: World Health Organization; 2017
Fake and counterfeit drugs are posing severe challenges in the developing countries like India where several studies alarmed about a thriving fake and counterfeit drug market in India. In other words, it’s a global problem which requires a collective and comprehensive response from the political leadership.
In this backdrop, World Health Organisation (WHO) published a comprehensive study on the global substandard and falsified medical product – WHO Global Surveillance and Monitoring System for substandard and falsified medical products (GSMS) which highlights the causes, consequences and solutions. This study paints a gloomy picture. In order to give a clear understanding of such challenges, the report stated that substandard and falsified medical products are most likely to be found where:
- Access to affordable, quality, safe and effective medical products is constrained.
- Standards of governance are low, ranging from poor ethical practices through to corruption in both the public and private sectors.
- The tools and technical capacity to ensure good practices in manufacturing, quality control and distribution are limited.
The existence of substandard and falsified (SF) medical products is an unacceptable risk to public health. They affect every region of the world, and medicines from all major therapeutic categories have been reported, including vaccines and diagnostics. The study on the public health and socioeconomic impact of substandard and falsified medical products, which examines estimates of the observed failure rates and spending.
Countries in which substandard and falsified medical products have been discovered and reported to the WHO GSMS: 2013–2017
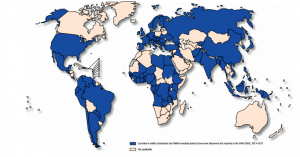
Source: WHO Global Surveillance and Monitoring System for substandard and falsified medical products, Geneva: World Health Organization; 2017
No country has the capacity to inspect tens of thousands of different medical product formulations coming from hundreds of different manufacturers. Often, the best they can do is to assume that if the product is manufactured in a well-regulated country, it will be of acceptable quality. That is generally a safe assumption, especially where cooperative agreements allow the importing regulator to carry out due diligence checks with regulators in producer countries.
Percentage of Reports from Each WHO Region to the GSMS (2013–2017):
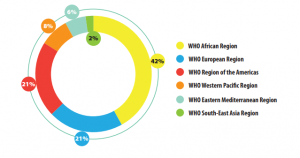
Source: WHO Global Surveillance and Monitoring System for substandard and falsified medical products, Geneva: World Health Organization; 2017
Despite this huge and continuing challenge, efforts to reduce global inequities in access to health care have succeeded at least partially. Per capita, spending on health more than doubled worldwide in the 20 years to 2014 (For More Detail, Please See the Report).
In low-income countries, spending on health care close to tripling over those two decades. Although much of that still comes out of the pockets of families who can ill-afford it, the percentage of the health care bill paid by governments rather than families is rising fastest in the poorest countries, as the report documented.
WHO has come up with a mechanism to deal with such challenges. As the report underlined, the global supply chains require a global system that can quickly alert people worldwide to the danger posed by substandard and falsified medical products. According to the report, medicines that fail to protect or cure patients strain the budgets of households and health systems, damaging the very fabric of society. Legitimate manufacturers of both generic and innovator pharmaceutical products suffer financially and reputationally when criminals falsify their products.


















